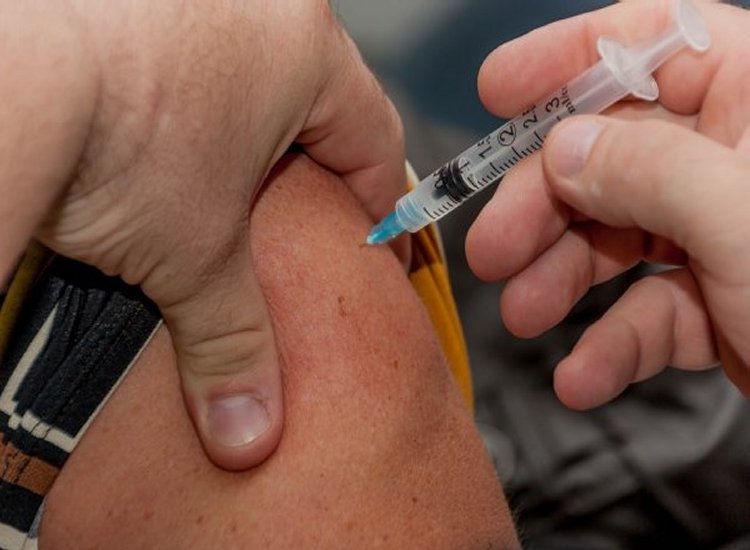
AstraZeneca announced that its Covid-19 vaccine provided strong protection among adults of all ages in a US based study. The study is being used to rebuild confidence in the mRNA vaccine and will help AstraZeneca to gaining US clearance for their drug.
The study involved 30,000 people and mould that the mRNA vaccine was 79% effective in preventing symptomatic cases of Covid-19.
However, according to Breakingnews.ie, hours after publishing the study results, the US National Institute of Allergy and Infectious Diseases said the DSMB “expressed concern that AstraZeneca may have included outdated information from that trial, which may have provided an incomplete view of the efficacy data”.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the statement added.
Tell us your thoughts in the Facebook post and share this with your friends.